OmniActive’s Meso-Zeaxanthin Granted Self-Affirmed GRAS Status
The expert panel evaluated the eye-health ingredient in accordance with FDA guidelines and found that it met the safety criteria.
Photo © iStockphoto.com/kutaytanir
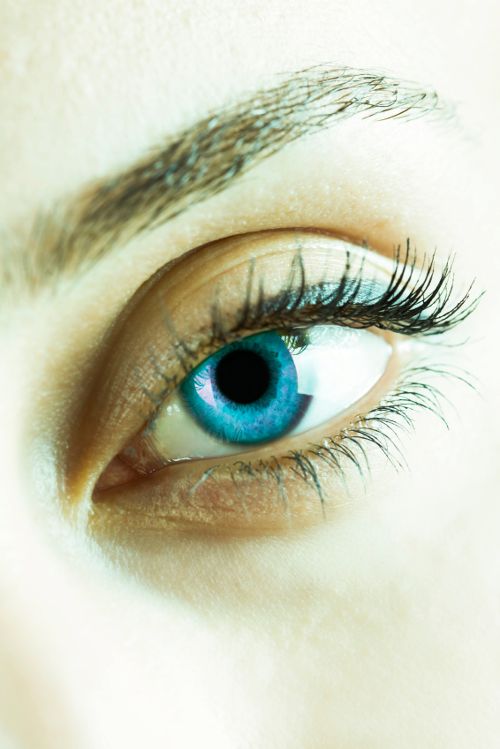
OmniActive Health Technologies (Morristown, NJ) has received a notification from a panel confirming the Generally Recognized as Safe (GRAS) status of its OmniXan-RS meso-zeaxanthin ingredient. The expert panel evaluated the eye-health ingredient in accordance with FDA guidelines and found that it met safety criteria. According to the company, the OmniXan-RS ingredient (meso-zeaxanthin, or 3R,3’S zeaxanthin) is made from marigold flower and is grown and extracted through a vertically integrated supply chain. “With the rising interest and science supporting meso-zeaxanthin, OmniXan-RS helps meet a gap for a standalone RS-zeaxanthin ingredient in the market,” said Lynda M. Doyle, senior vice president of global marketing, OmniActive, in a press release. “Like most of the ingredients in our portfolio, we obtained GRAS self-affirmation to further position OmniXan-RS as unique and safe.” In addition to OmniXan-RS, the company’s range of macular carotenoids includes Lutemax 2020 lutein with enhanced zeaxanthin, Lutemax Free lutein, and OmniXan RR-zeaxanthin, made from natural paprika. Also read: Kemin's ZeaOne Zeaxanthin Gets FDA GRAS Lutein and Zeaxanthin Elicit "Rapid Retinal Response," Study Suggests The Blue-Light Message: Explaining the Benefits of Lutein and Zeaxanthin
HHS announces restructuring plans to consolidate divisions and downsize workforce
Published: March 27th 2025 | Updated: March 27th 2025According to the announcement, the restructuring will save taxpayers $1.8 billion per year by reducing the workforce by 10,000 full-time employees and consolidating the department’s 28 divisions into 15 new divisions.






