Nutrition Facts Label Ingredients: 2015 Ingredients to Watch for Food, Beverage, Supplements
Nutrients facing potential changes under FDA's Nutrition Facts label proposal are definitely one of 2015's ingredients to watch. Check back daily as we release new predictions.
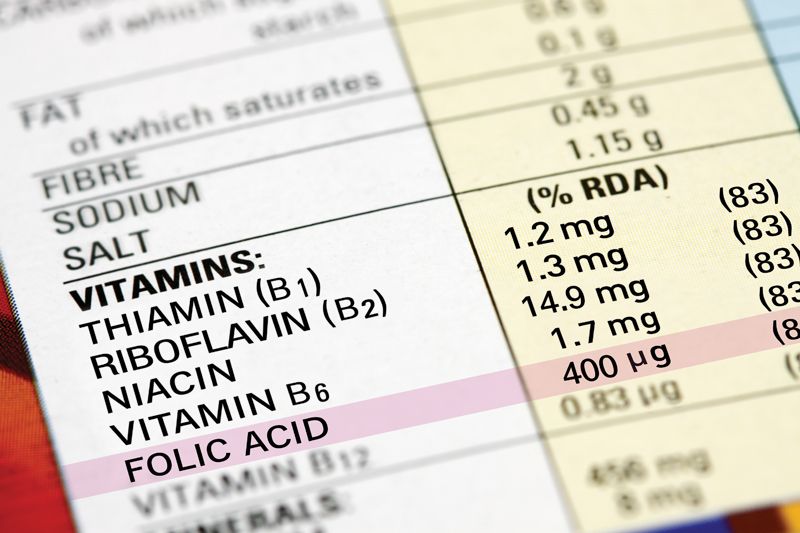
FDA’s proposed changes to the Nutrition Facts label will likely significantly impact food manufacturers and some specific nutrients especially. While public reception is overall positive to some changes, such as a switch to using updated Daily Values and making vitamin D and potassium listings mandatory, other changes are more controversial. It will be interesting to see FDA’s takeaways as it sifts through all of the public comments submitted last August.
Sugar
Without a doubt, nutrition label debate is hottest over sugar. FDA proposes not only declaring total sugar content, but also, on a separate, indented line, now also specifying the amount of added sugar. FDA’s thinking is that by revealing high amounts of extra, added sugar in some foods (sugar added during food processing), it will motivate consumers to instead choose foods with natural sugars that may be more nutrient dense. Groups like the American Heart Association and the American Diabetes Association support this labeling change. But would the revised Nutrition Facts label actually change consumer behavior? The data backing this cause-and-effect assumption is far from set in stone. In its comments to FDA, the International Food Information Council (IFIC) said that in a 2014 IFIC consumer test of the newly proposed label, many consumers did not understand that the added-sugar value is a subset of total sugar, instead believing it represents additional sugar content.
Will sales of packaged foods plummet once their added-sugar content is exposed? That’s what’s keeping the food industry awake at night. Expect further resistance.
Fiber
Fiber is another ingredient mandatory on the Nutrition Facts label that could see significant changes under the new proposal. FDA currently has no formal definition for fiber, but it now proposes adopting a definition developed by an IOM panel in 2001. Dietary fiber, as listed on the Nutrition Facts label, would include non-digestible soluble and insoluble fibers naturally present in fruits and veggies. It could also include other fibers, such as an isolated non-digestible fiber that has at least three monomeric units and that FDA deems beneficial to human health-but FDA must approve their inclusion, and companies would have to first petition FDA or seek a health claim from the agency. FDA would still allow both soluble and insoluble fibers to be voluntarily declared.
The proposed definition for now leaves some out in the cold. FDA says it currently only considers two synthetic soluble fibers to be dietary fiber: beta-glucan, like in oatmeal, and barley beta-fiber. Others, such as non-digestible oligosaccharides like inulin and maltodextrin which today are often added to foods for their prebiotic value, are excluded, and this may cause an uproar in the prebiotic market.
FDA’s proposals will also make it harder for companies to hit the high-fiber mark and make “high fiber” claims. The agency proposes increasing the daily reference value (DRV) for dietary fiber from 25 g to 28 g, and companies may struggle if they can no longer factor in fibers now excluded from FDA’s definition.
Folic Acid
Consensus on the importance of folic acid in preventing neural tube defects (NTDs) in the developing fetus is by now pretty much universal, and making sure that women across the globe understand this crucial message is a priority for authoritative bodies worldwide. Last November, the European Union approved a health claim linking folic acid intake with reduced risk of NTDs.
In the United States, companies have been able to voluntarily list folate content. (Folate is the vitamin B9 found naturally in food. Folic acid is the synthesized version used in dietary supplements and food fortification.) FDA’s new proposal maintains this voluntary listing; however, there are two important changes on the table.
First, FDA proposes only allowing supplements to use the termfolic acid and foods to use the term folate. The problem, the Council for Responsible Nutrition (CRN; Washington, DC) pointed out in its comments to FDA, is that many supplement companies include both folic acid and synthetic folate in their products. Labels won’t be accurate if supplement companies are prevented from disclosing the complete folic acid/folate content in their products.
A second proposed change is even more concerning. While folate is currently listed in microgram (µg) units on today’s nutrition label, FDA suggests changing the units of measure to what’s known as dietary folate equivalents (DFE) in order to capture the difference between natural folate from food and folic acid from food and supplements. The problem is that the two units of measure are not equivalent. In many parts of the world, 400 µg folic acid is the intake recommended and supported for preventing NTDs. But 400 µg DFE of folic acid is equal to less than 400 µg folic acid-only 235 µg, actually.
As CRN points out, there could be two results. First, companies could lower the level of folic acid in their product to 400 µg DFE, and as a result, women may end up getting less folic acid. “FDA adoption of the change in the unit of measure would mean that a lower level of folic acid would be represented in the Nutrition Facts labels of fortified foods as providing 100% of the DV for folate. Women would be unaware that 100% of the proposed DV does not provide the full 400 µg of folic acid recommended for protection against NTDs,” CRN wrote. Alternatively, it points out, companies could still continue providing 400 µg worth of folic acid in product, but this would now appear to be in excess of the Recommended Dietary Allowance (RDA).
With so much at stake, it will be crucial that any change FDA ultimately makes to the Nutrition Facts label does not create confusion among women regarding how much folic acid they should take.
Choline
Like folic acid, choline is crucial to fetal development. In adults, choline also plays an important role in brain health, liver health, heart health, and muscle performance. Despite this, at least 90% of Americans are deficient in this essential nutrient. The Institute of Medicine (IOM) recognized this in 1998, and the 2010 Dietary Guidelines for Americans advisory committee cited choline as a “shortfall nutrient.” The hope is now that the 2015 Dietary Guidelines for Americans, when released, will include advice to increase choline intake in the United States.
For the first time, we might also see choline listed on FDA Nutrition Facts labels. In its proposed changes, FDA suggested adding choline to the list of nutrients permitted to be voluntarily listed on the Nutrition Facts label. If choline is allowed on the label, it could help to spark widespread awareness of the importance of this crucial nutrient, says the Choline Information Council, a group started last year to spread the word.
Also read:
Five Biggest Struggles with FDA’s New Nutrition Label (Slideshow)
2015 Ingredients to Watch
Photo © iStockphoto.com/Empato
The Nutritional Outlook Podcast Episode 36: Best of the Industry Service Provider, Radicle Science
December 26th 2024Nutritional Outlook's managing editor, Sebastian Krawiec, interviews Radicle Science co-founders, Pelin Thorogood and Jeff Chen, MD. Radicle Science has been selected as this year's Best of the Industry, Service Provider.