Probiotic and herbal blend for respiratory health shown safe for use in healthy subjects
Researchers from the University of Alabama at Birmingham conducted the trial with contract research organization Atlantia Clinical Trials.
Photo from Atlantia Clinical Trials

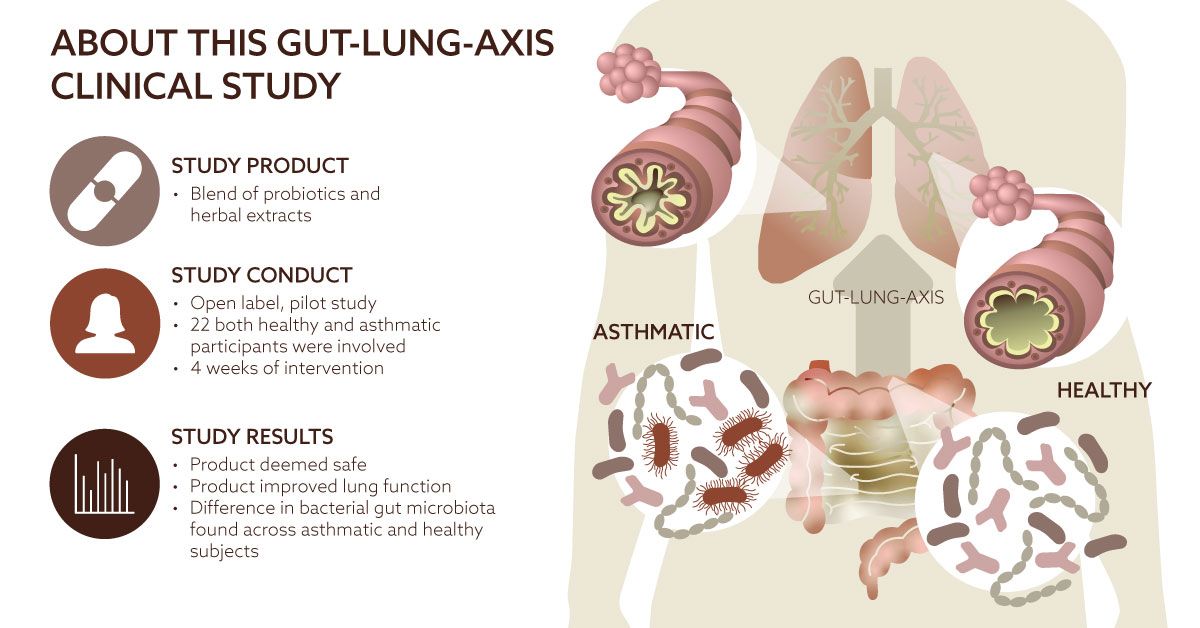
A probiotic-and-herbal supplement for respiratory health, called ResB, was shown in a recent human study to be safe for use. ResB was developed by ResBiotic Nutrition, which is a university startup out of the University of Alabama at Birmingham. University researchers, along with contract research organization Atlantia Clinical Trials (Cork, Ireland), conducted the one-month, open-label pilot study (ClinicalTrials.gov Identifier: NCT05173168). Funding for the study was provided by the National Institutes of Health, and the ResB supplement was donated by Resbiotic Nutrition Inc.
The study was conducted on 22 male and female subjects aged 18-65 who were generally healthy—and half of whom had asthma and had been on stable medication for the condition for at least three months. Twice per day during the trial, all subjects took one capsule of the ResB supplement. The supplement blends together the probiotic strains 8.25 x 109 CFU Lactobacillus plantarum RSB11, 7.9 x 109 CFU Lactobacillus acidophilus RSB12, and 6.4 x 109 CFU Lactobacillus rhamnosus RSB13, with the following herbal extracts: 48 mg of vasaka root extract (Adhatoda vasica), 42 mg of holy basil extract (Ocimum sanctum), and 30 mg of turmeric extract (Curcuma longa).
During the study, researchers primarily studied safety endpoints, but they also looked at exploratory endpoints such as quality of life, lung function, gut microbiome ecology, and inflammatory biomarkers. Throughout the study, the researchers gathered data using 16s gene sequencing, stool samples, changes in lung function measured by spirometry, changes in oxygen levels, and forced vital capacity. Subjects were also asked to complete the Saint George’s Respiratory Questionnaire.
By the end of the study, “All subjects tolerated the blend without adverse events,” the researchers reported.
Moreover, in this study, subjects who had asthma showed significant improvement in lung function “as measured by forced expiratory volume and serum short-chain fatty acid levels from baseline to week 4,” they wrote. (Forced expiratory volume is the amount of air a person can force out of their lungs in one second. And bacterial species such as L. rhamnosus, L. plantarum, and L. acidophilus have been shown to help maintain proper uptake of short-chain fatty acids. When short-chain fatty acids and other metabolites produced by commensal bacteria travel throughout the body, they help mediate inflammatory and immune responses in the body, the researchers explained.) They also noted the potential anti-inflammatory effects of the herbal extracts in the blend, which they said could support antioxidant, antitussive, anti-inflammatory, and bronchodilatory effects in the lungs and systemically.
Patients who have respiratory diseases often exhibit imbalanced gut microbiomes compared to healthy people, the researchers pointed out, noting that “the state of the intestinal microbiota is deeply connected to lung health and vice versa.” In the current study, researchers observed that “The gut microbiome of asthmatic subjects differed significantly from controls, with the most prominent difference in the relative abundance of the proteobacteria Escherichia coli.”
The gut-lung axis is thought to play an important role in respiratory health. As the researchers state in their report, “Dysbiosis of the gut microbiome may augment lung disease via the gut-lung axis. Proteobacteria may contribute to tissue proteolysis followed by neutrophil recruitment, lung tissue injury, and perpetuation of chronic inflammation. To study the effects of probiotics across the gut-lung axis, we sought to determine if a Lactobacillus probiotic and herbal blend was safe and well-tolerated in healthy volunteers and asthmatic patients.”
The researchers concluded that “This study supports the safety and efficacy potential of a Lactobacillus probiotic plus herbal blend to act on the gut-lung axis.” They further recommended carrying out longer-term subsequent trials with control groups, placebo control, and blinding.
Polyphenols: The next generation of prebiotics is ready for liftoff
April 21st 2025Explore the prebiotic health benefits of polyphenols and the positive impact they may have on digestive and immune health. Polyphenols, such as those found in European black elderberry, may be an ideal solution for manufacturers trying to break into the digestive health space.










