NOW testing shows potency far below label claims in certain products purchased on Amazon
NOW has publicized the results of a series of internal tests it had conducted on CoQ10 and SAMe products purchased on Amazon.com. Results showed that the potencies of the products were far below that of label claims.

Figure 1. CoQ10 Test Results
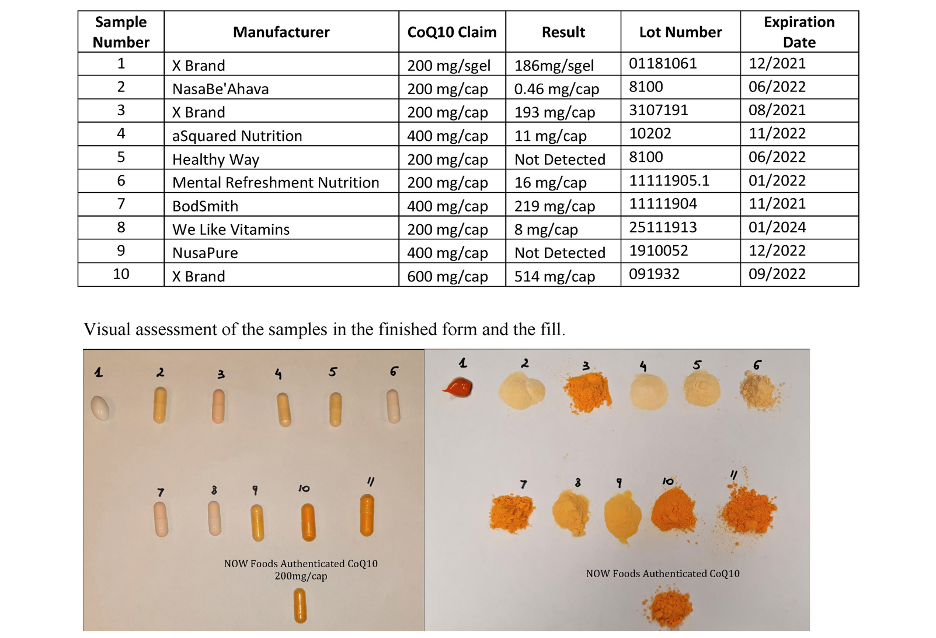
Figure 2. SAMe Test Results
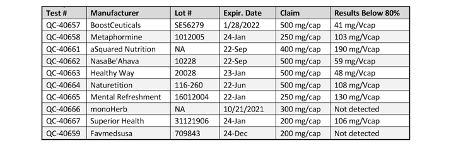
NOW (Bloomingdale, IL) has publicized the results of a series of internal tests it had conducted on CoQ10 and SAMe products purchased on Amazon.com. Results showed that the potencies of the products were far below that of label claims.
“Today, especially, more people are buying their supplements online, which is why we are making this information public,” said Dan Richard, NOW’s vice president of global Sales and marketing, in a press release. “NOW takes defrauding consumers personally and it is in the best interest of the entire dietary supplements to identify and work to purge such bad actors to protect consumers.”
NOW tested ten CoQ10 products and ten SAMe products using High Performance Liquid Chromatography (HPLC) in its labs using validated methods. Of the CoQ10 products, six of the ten brands contained less than 20% of the labeled potency, two of which had no detectable CoQ10 at all (See Figure 1).
Analytical testing of the SAMe products also showed potencies well below the labeled claims. Because SAMe is typically unstable in heat and moisture, requiring an enteric coating in tablets to stabilize, or the use of the form S-Adenosyl-L-Methionine from disulfate tosylate salt, NOW speculates that the products were either low potency or in unstable form (See FIgure 2).
“As a business partner of Amazon, we did report this information to them and hope they will take action,” said Richard. “Additionally, NOW has provided this information to other supplement brands, FDA, and to trade associations.”
Prinova acquires Aplinova to further increase its footprint in Latin America
April 7th 2025Prinova has recently announced the acquisition of Brazilian ingredients distributor Aplinova, which is a provider of specialty ingredients for a range of market segments that include food, beverage, supplements, and personal care.










