Non-Animal Chondroitin Sulfate Ingredient Mythocondro Gets FDA GRAS
Mythocondro, a non-animal-sourced chondroitin sulfate ingredient from Gnosis S.p.A. (Desio, Italy), has received a “no objections” response from FDA for Generally Recognized as Safe (GRAS) status for safe use in food and beverage.
Photo © iStockphoto.com/Raycat
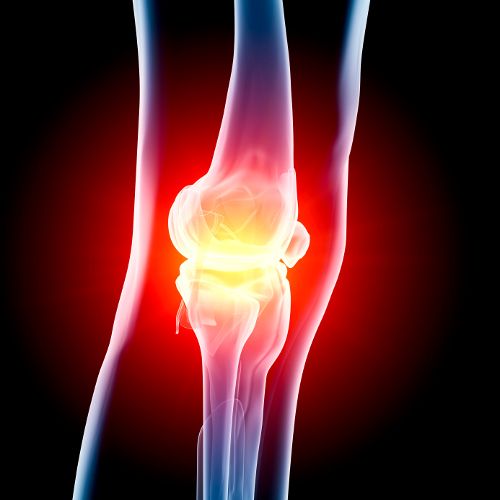
Mythocondro, a non-animal-sourced chondroitin sulfate ingredient from Gnosis S.p.A. (Desio, Italy), has received a “no objections” response from FDA for Generally Recognized as Safe (GRAS) status for safe use in food and beverage.
The ingredient is produced via a fermentation-based manufacturing process. Due to its origins, the company contends that Mythocondro sidesteps product-of-origin and adulteration questions that face much of the animal-source chondroitin sulfate market. The ingredient was introduced last fall during SupplySide West. The ingredient is also a finalist in this year’s NutraIngredients Awards, which will be announced during the upcoming Vitafoods Europe trade show in May.
Magnesium L-threonate, Magtein, earns novel food authorization in the European Union
December 19th 2024According to the announcement, the authorization is also exclusive to AIDP and its partner company and licensee, ThreoTech, meaning that they are the only parties that can market magnesium L-threonate in the EU for a period of five years.
Senate Committee has released the text of 2024 Farm Bill, with changes to hemp regulations
November 19th 2024The U.S. Senate Committee on Agriculture, Nutrition, & Forestry has introduced the Rural Prosperity and Food Security Act, which will serve as the Senate’s draft for the 2024 Farm Bill.