Liposomes and nutraceuticals: Crushing bioavailability barriers
Liposomal delivery systems have been used to change the pharmacokinetic profiles of herbs, vitamins, enzymes, and even drugs to improve overall therapeutics.
Bioactives and nutraceuticals have become an integral part of our lives. If there’s one positive to come from the COVID-19 pandemic, it is an increased awareness about general health and well-being. To boost immunity and fill nutritional gaps in the diet, the nutraceuticals market has been flooded with supplements like vitamin C, glutathione, zinc, and curcumin. But do we know whether or not these high-value supplements are actually delivering therapeutic benefits?
No doubt, supplements like vitamin C, coenzyme Q10, and calcium are essential for regular metabolism. When we take these as supplements in conventional forms, however, they can be either poorly soluble or poorly permeable, or can get damaged by oxidation or peroxidation—all in all reducing their bioavailability in the body.1 Often, increased supplement dosages are suggested to improve bioavailability, but this also poses the risk of associated side effects.
Liposomal Benefits
Liposomal delivery systems have been used to change the pharmacokinetic profiles of herbs, vitamins, enzymes, and even drugs to improve overall therapeutics.2,3
Phospholipids are the main components of liposomes and have the ability to self-assemble to generate different supramolecular structures like liposomes, micelles, etc.3,4 Due to phospholipids’ amphiphilic nature, both water-soluble and fat-soluble actives can be loaded easily into phospholipid matrices.
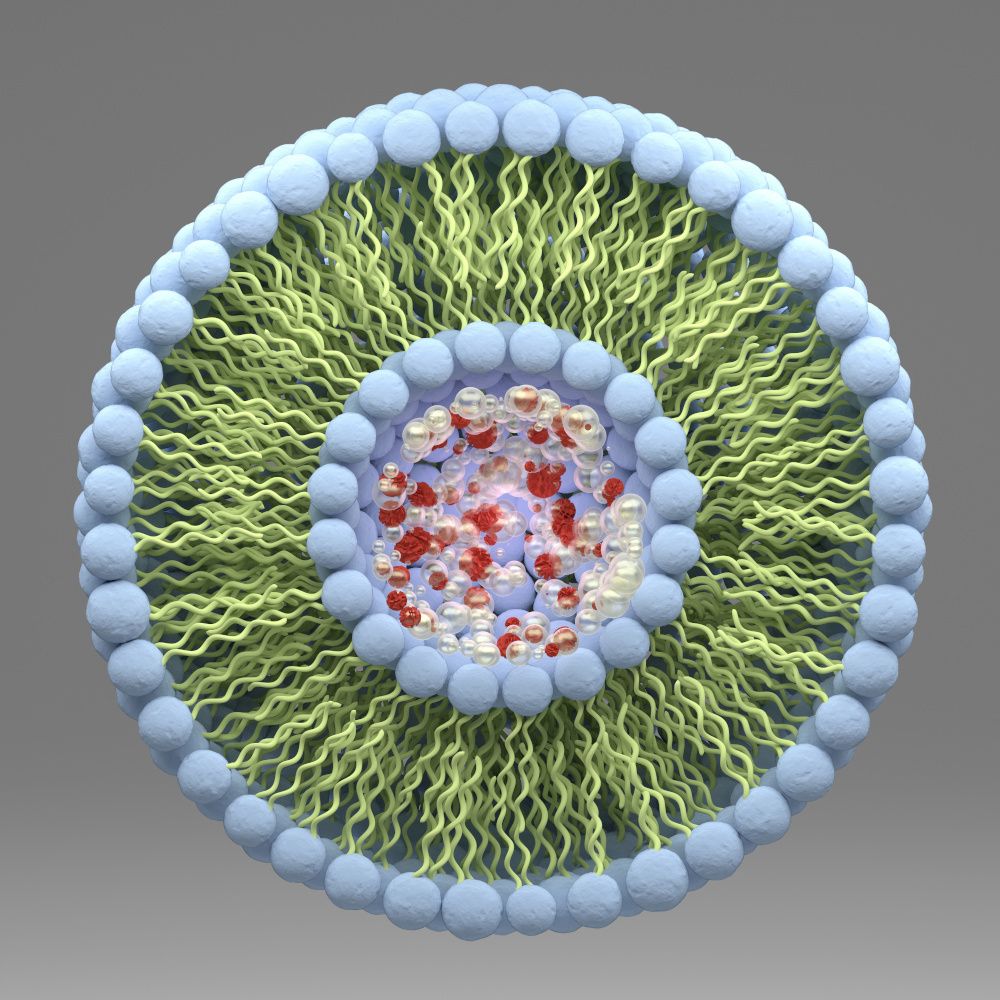
Liposomes are nanoparticles or nanocarriers, usually 100-300 nm in diameter. They are formed spontaneously when certain phospholipids are hydrated with water.3,4 Liposomes are microscopic lipid vesicles.3,4 These mimic the human cell membrane. Because of their unique properties, liposomes are able to enhance the performance of products by increasing solubility and improving bioavailability and stability of active ingredients.3,7
How Liposomes Work
During first-pass metabolism, typically there is a reduction or slowdown in nutrient bioavailability due to the presence of digestive enzymes, acidic pH of the stomach, and bile salts.
Once active ingredients are loaded onto a liposome, however, they can easily pass through first-pass metabolism. Liposomes enable drugs and/or nutrients to navigate the lymphatic pathway, bypassing the hepatic first-pass effect.5 They not only protect the bioactive but also prevent any nutritional loss, help incorporate any pulsatile or time-release mechanisms in the formulation, and mask and preserve the actives as well.
Once the liposomes reach the small intestine intact, they adhere to the cells of the small intestine. The small intestine cell wall is rich in phospholipids as well. The two main pathways that are suggested to enhance oral drug delivery by liposomes are via drug release in the gastrointestinal lumen or via transformation of vesicles into mixed micelles, and subsequent permeation of drug molecules across the intestinal epithelia.6
Since the liposomes mimic the cell membrane, there is no metabolic load on the liver. Higher nutrient doses are not required because of efficient and accurate delivery by the liposomes.
What Are Manufacturing Techniques for Liposomes?
The quality of liposomes significantly depends on the manufacturing technique. To develop stable liposomes with high encapsulation efficiency, methods like ethanol injection, thin-film hydration, reverse-phase evaporation, and more-modern methods such as microfluidics can beused to scale up industrially. These methods ensure higher encapsulation efficiency and effective drug loading and help to form smaller and uniform-size vesicles, resulting in increased permeation and bioavailability compared to standalone micronutrients.
In all, liposomes can be a promising delivery system because of their biocompatibility, biodegradability, and low toxicity in the human body7 and should be adopted more often to meet the real needs of today’s nutrition industry.
Aditi Pawar, executive, scientific business development (nutrition), and Vrushali Patil, marketing manager, represent VAV Lipids (Mumbai, India), an innovation-driven company specializing in cGMP-grade manufacturing of lecithin and phospholipids for nutraceutical, pharmaceutical, and cosmeceutical applications. For more information, please visit: http://www.vav.in/ or e-mail marketing@vav.in.
References
- Caritá AC et al. “Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability.” Nanomedicine: Nanotechnology, Biology, and Medicine. Published online October 30, 2019. Accessed here.
- Lee M et al. “Liposomes for enhanced bioavailability of water-insoluble drugs: in vivo evidence and recent approaches.” Pharmaceutics, vol. 12, no. 3 (March 13, 2020):264. Accessed here.
- Kidd P. “Phospholipids: Versatile nutraceuticals for functional foods.” Journal of Nutraceuticals, Functional & Medical Foods. (January 2002) Accessed here.
- Li J et al. “A review on phospholipids and their main applications in drug delivery systems.” Asian Journal of Pharmaceutical Sciences, vol. 10, no. 2 (April 2015): 81-98. Accessed here.
- Ahn H et al. “Liposomal delivery systems for intestinal lymphatic drug transport.” Biomaterials Research. Published online November 23, 2016. Accessed here.
- Haisheng He et al. “Adapting liposomes for oral drug delivery.” Acta Pharmaceutica Sinica B, vol. 9, no. 1 (January 2019): 36-48. Accessed here.
- Keller BC. “Liposomes in nutrition.” Trends in Food Science & Technology, vol. 12, no. 1 (January 2001): 25-31. Accessed here.
- Wei Wu et al. “Oral delivery of liposomes.” Therapeutic Delivery, vol. 6, no. 11 (2015): 1239-1241. Accessed here.