CRN, CHPA, and UNPA has submitted to FDA a proposed framework for the NDI master file
In a joint letter to FDA, the industry trade groups propose a framework for FDA to implement a New Dietary Ingredient (NDI) master file and urge clarification on the agency’s views in a final NDI guidance.

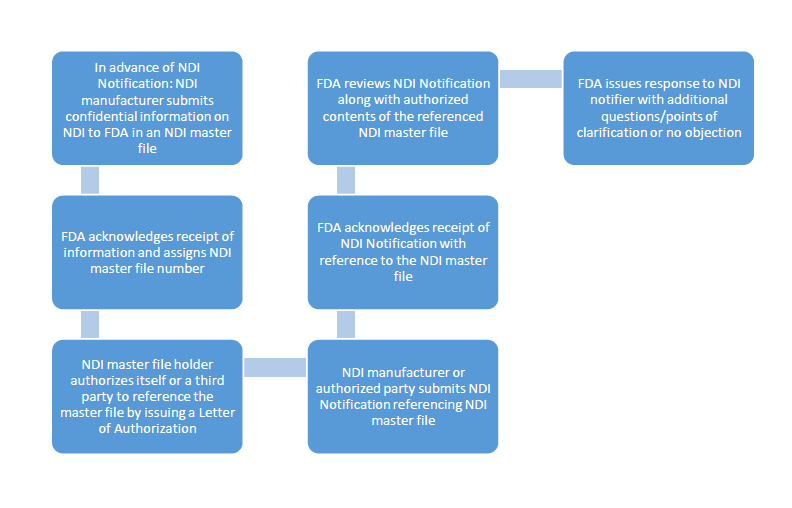
Figure 1: Schema of the process of submitting an NDI master file and a subsequent NDI notification
In a joint letter to Steve Tave, director, Office of Dietary Supplements Programs, Center for Food Safety and Applied Nutrition at U.S. Food and Drug Administration (FDA), the Consumer Healthcare Products Association (CHPA; Washington, D.C.), the Council for Responsible Nutrition (CRN; Washington, D.C.), and the United Natural Products Alliance (UNPA; Salt Lake City, UT) propose a framework for FDA to implement a New Dietary Ingredient (NDI) master file and urge clarification on the agency’s views in a final NDI guidance. The goal of the NDI master file is to protect intellectual property.
“Responsible companies in the dietary supplement industry invest in generating the necessary data to establish the safety of their ingredients,” states the letter. “A significant concern for these ingredient manufacturers is that they must compete on an uneven playing field with companies that fail to generate and submit safety data specific to their NDIs and claim that their ingredients are identical to NDIs that other firms properly notified.”
Unfortunately, it is often not the case that ingredients are identical to existing NDIs, sometimes posing a safety risk to consumers. According to the letter, the lack of deterrence for unscrupulous companies bypassing the notification requirements only frustrates and discourages responsible companies from the NDI notification process, potentially stifling innovation. The NDI master file would be a tool to streamline the collection and protection of data investments made by ingredient manufacturers.
The way it would work is that in advance of an NDI notification, a manufacturer would submit confidential information (trade secrets) on the NDI to FDA in an NDI master file. FDA would acknowledge receipt of the information and assign an NDI master file number. FDA would not, however, approve or disprove the submission of the master file. FDA would only review the master file information in the context of an NDI notification under 21 CFR 190.6. The NDI master file holder would then be able to authorize oneself or a third party to reference the master file in an NDI notification by issuing a Letter of Authorization (see figure 1).
The proposed framework is modelled after the U.S. Drug Master File, which has been accepted by FDA for decades. It was developed by the members of CRN’s Master File Working Group, and has been reviewed by CHPA and UNPA.
“As partners in this industry effort, we jointly submit the proposed framework to encourage and assist FDA in implementing an NDI-MF system that will serve both the industry and the agency,” concludes the letter. “We underscore, however, that the utility, and ultimate success, of the NDI-master file will depend largely on FDA’s support and a strong commitment to enforcement of its proper use-establishing a master file program without the agency’s resolve and resources to enforce it, and to prosecute companies who ignore it, only perpetuates continued indifference and leaves the intellectual property of responsible and innovative companies unprotected.”
HHS announces restructuring plans to consolidate divisions and downsize workforce
Published: March 27th 2025 | Updated: March 27th 2025According to the announcement, the restructuring will save taxpayers $1.8 billion per year by reducing the workforce by 10,000 full-time employees and consolidating the department’s 28 divisions into 15 new divisions.






