Claims Crisis?
When the Food and Drug Administration (FDA) slapped General Mills with a stern warning letter over its Cheerios heart-health claims last May, it signaled the start of increased scrutiny by the agency of misleading food labels.
Originally Published NO March 2010

When the Food and Drug Administration (FDA) slapped General Mills with a stern warning letter over its Cheerios heart-health claims last May, it signaled the start of increased scrutiny by the agency of misleading food labels.
FDA's is not the only radar that's perking up, it seems. Thanks to subsequent complaints over other dubious claims, such as Kellogg's "Now helps support your child's immunity" line on Cocoa Krispies, consumers are beginning to realize just how far from FDA's legal line some companies-including industry giants-may have strayed.
Public outcry was especially loud over the Smart Choices front-of-package labeling program. Funded by the food industry, the program touted what critics called lax nutrition criteria, and eventually ended up suspending itself last fall, not long after its summer debut. In the media, critics did not mince words. "The object of this is to make highly processed foods appear as healthful as unprocessed foods, which they are not," said Marion Nestle, a New York University nutrition professor, to The New York Times.
In October, FDA stepped into the fray. FDA commissioner Margaret Hamburg announced that the agency would begin actively policing misleading front-of-package nutrition labels. Hamburg also declared that FDA would embark on establishing official front-of-package nutrition symbol guidelines-marking the first time the agency has done so.
In her speech, Hamburg didn't quite use the words "enough is enough." However, she did liken questionable food claims to "a 'Tower of Babel' on food packages proclaiming natural and, sometimes, disease-related health benefits that were either unfounded or misleading."
DESIGNS FOR THE NUTRITION FACTS LABEL
IN MARCH, the Center for Science in the Public Interest (CSPI; Washington, DC) released the final version of its report titled "Food Labeling Chaos: The Case for Reform." The report contains a number of proposals, aimed at FDA, for how CSPI believes food labeling should be amended.
CSPI's widespread suggestions cover everything from ceasing misleading health claims to revising regulations for the Nutrition Facts panel-including declaring a product's caffeine content, not allowing "isolated" fibers to count towards fiber content, and making ingredient labels easier to read by establishing new rules for type size, fonts, and how ingredients such as added sugars are listed. (Visit CSPI's website, www.cspinet.org, to download the full report.)
Much of what CSPI proposes would require changes to existing labeling regulations under the Nutrition Labeling and Education Act (NLEA). Elizabeth Campbell, vice president of EAS Consulting Group (Alexandria, VA), was part of the FDA task force that wrote and implemented NLEA's regulations. She says that when her team was drafting the regulations, it did discuss some of the issues that are still on the table in CSPI's report today.
Campbell adds that for some issues, the NLEA committee lacked the time, resources, and in some cases, reason and evidence, to lay down certain regulations in the relatively short amount of time it was given to draft the regulations. For instance, she says, the team was not able to adequately define the term natural in a way that wouldn't have had "negative consequences" when the term is used in the marketplace. Also, she says, in the late 1990s, when CSPI originally requested that FDA require a quantitative declaration of caffeine content on labels, scientific evidence was lacking to support a health basis for such a requirement at the time.
It's difficult to say whether today's FDA has the resources to address the issues that CSPI brings up. However, FDA has opened the door for some labeling reform. In addition to creating standards for front-of-package symbols, the agency is currently considering making updates to the Nutrition Facts label, include revising how calorie contents, serving sizes, and Daily Values are listed.
Silverglade says that he believes that even if FDA does step up enforcement on misleading labeling, regulatory changes are still needed. "I think FDA has to follow up warning letters with industry-wide enforcement through rulemaking," he says. "We would like the agency to control the misleading claims we've identified by issuing regulations for the industry that clearly set up the parameters of what can and can't be said on food labels. So far, the agency has been addressing the problem on a case by case basis, picking out the most egregious example and targeting a specific company."
He continues, "The agency is flexing its muscle, showing that this issue is back on the radar screen and that it's serious about reforms such as front-of-package nutrition labeling. We don't know how fast or how much the agency will do, but it certainly seems to be moving in that direction. We wanted to make sure that we gave FDA our blueprint for what we think should be done overall."
Many seem to welcome the idea of a more-active FDA in the labeling arena. And for the first time in many years, it may happen.
Absence Makes the Claims Grow Bolder
In her keynote address to the National Food Policy Conference last September, Hamburg said, "[Food labeling] is an issue that's critical for the health and vitality of our nation, and yet it is a concern that has not been substantially addressed since the FDA implemented the Nutrition Labeling and Education Act [NLEA] more than 16 years ago."
That act was passed in 1990 (and went into effect in 1994) to try to stop the misleading labeling that had become "a major problem" by that time, says Bruce Silverglade, director of legal affairs for nonprofit health advocate Center for Science in the Public Interest (CSPI; Washington, DC). Among other things, the NLEA introduced the Nutrition Facts panel and defined terms such as low fat and light. The law also authorized a handful of specific FDA-approved health claims that could be used for foods.
According to Silverglade, misleading food claims continued to happen in spite of NLEA rules, in part because of lack of enforcement of those rules by FDA-especially under the previous administration. "FDA took various measures to stop enforcing the law," he says. "When one company gets away with breaking the law, competitive pressures cause other companies to fall to the lowest common denominator, leading the entire industry on a downward spiral."
Elizabeth Campbell, vice president of EAS Consulting Group (Alexandria, VA), was part of the FDA task force that wrote NLEA's regulations. She agrees that FDA labeling enforcement had become somewhat soft. "During the Bush administration, the pendulum swung pretty far to the 'don't-do-much' side. I've always thought that you need adequate enforcement, and when we did not have enough enforcement, I was frustrated. Whenever FDA is absent in an area, the marketplace takes over."
On March 3, in a letter to industry, Hamburg herself noted the recent increase in labeling infractions. "We continue to see products marketed with labeling that violates established labeling standards," she said, calling such labels "false" and "misleading."
Claims Creeping In
Supporting the immune system has become a more common health target, resulting in numerous appropriate structure/function claims that FDA currently considers legal. Unfortunately for Kellogg's, the launch of its questionable "Now helps support your child's immunity" phrase on boxes of Cocoa Krispies coincided with the global spread of swine flu-which sparked San Francisco city attorney Dennis Herrera's letter to Kellogg's expressing concern that "the immunity claims may also mislead parents into believing that serving this sugary cereal will actually boost their children's health."
In the case of Cheerios, FDA's letter to General Mills stated that some claims misbranded the product, while others made claims, such as cholesterol-lowering statements, that are only allowed for drugs. FDA took issue with wording such as "you can lower your cholesterol 4% in 6 weeks" and "...Cheerios is...clinically proven to lower cholesterol." FDA said that such marketing implied that Cheerios is intended for "preventing, mitigating, and treating the disease hypercholesterolemia."
FDA also chastised Cheerios for not using FDA-approved health claims properly or in their entirety. For instance, had Cheerios used FDA's sanctioned "Diets low in saturated fat and cholesterol and rich in fruits, vegetables, and grain products that contain some types of dietary fiber, particularly soluble fiber, may reduce the risk of heart disease, a disease associated with many factors" statement, instead of "Heart-healthy diets rich in whole grain foods can reduce the risk of heart disease," the company would have been deemed compliant.
HOW MANY CALORIES ARE IN THAT SODA?
SUGARY SODAS are generally not considered a healthy food. However, in an effort to make calorie information more apparent, leading nonalcoholic beverage companies have banded together to start printing calorie information on the fronts of beverage packages, reported the American Beverage Association (ABC; Washington, DC).
ABC says that the voluntary initiative is geared toward meeting First Lady Michelle Obama's request for "innovative industry initiatives that contribute to her healthy families program." The new calorie labeling is expected to take effect in the United States this year and be fully implemented by 2012. Coca-Cola has said that it expects its packaging to be relabeled by the end of next year.
ABC also notes that the initiative goes "above and beyond what is required by [the Food and Drug Administration's] food-labeling regulations." There's no word yet on how the new labeling scheme might fit in with the guidelines that FDA is currently working on for front-of-package labeling-or with FDA's stated intent to clear up consumer confusion caused by the numerous independent front-of-package labeling programs on the market. ABC notes that the beverage industry is working with FDA to implement the new calories-listing program.
FDA's heart disease claim may not sound as "sexy" as the more-succinct Cheerios claim. However, it seems straightforward enough. Moreover, it provides a more-complete picture of factors that contribute to good health. Why, then, do some companies eschew FDA's wording for their own?
Trying to stand out from the competition is one reason. Another reason? Because they have been allowed to, says Silverglade. In its defense, General Mills argued that it had been using the Cheerios statements for more than two years-clearly reflecting a lack of enforcement in this area. Perhaps it's also worth noting that the "Lower your cholesterol 4% in 6 weeks" claim was printed large, front and center, on the Cheerios box, as was the Cocoa Krispies immunity claim on its box.
Mostly, however, companies are willing to tread a thin line because it's known that such claims may seduce the consumer into buying. Recent consumer research conducted by ingredient supplier Beneo-Orafti (Tienen, Belgium) found that even in a down economy, consumers were still willing to pay more for premium-priced functional foods if they understood and valued those products' associated health benefits. (The survey addressed only legal structure/function claims for foods, not misleading health claims.)
In the online survey conducted last July on 1000 U.S. and German adults, consumers rated their top-four health-benefit claims of interest, which were calcium-absorption claims and claims of "protects your heart," "keeps your digestive system healthy," and "reinforces natural defenses."
CLAIMS RESONATE WITH CONSUMERS
IN THE WIDE WORLD OF FOOD CLAIMS, products touting omega-3s showed the highest sales growth in 2009, says The Nielsen Co. In its latest assessment of food sales based on health-benefit marketing, Nielsen found double-digit sales growth for a number of other claims as well. (See the table)
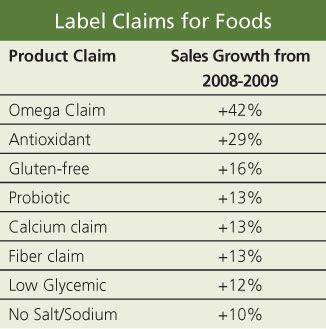
"Consumers in the U.S. might be trimming the fat from their budgets and diets, but contrary to predictions, they continue to demonstrate a healthy appetite for foods featuring health and wellness claims," says Tom Pirovano, director of industry insights for Nielsen.
Longstanding health claims also fared well. In particular, products with natural claims grew 4% in sales from 2008, outselling organic products by more than 4:1. Sales of reduced-calorie products grew 6%, while sales of products that claimed absence of a specific fat or with a preservative claim grew 1%. Sales of products with a sodium claim remained the same.
One claim that didn't fare as well was "lowers cholesterol." Sales of these products declined 5%. Nielsen suggests that perhaps an increase in popularity of statin drugs might account for the decline. Low-carb product sales also dropped 5%, while sales of products with soy claims were 6% lower.
Beneo-Orafti said that respondents indicated they would likely pay more for products with additional perceived health benefits. For instance, "In the U.S. survey...79% of all respondents were more likely to buy a well-known cereal product that had a suggestion of a calcium benefit on the packaging, and 60% of them would be prepared to spend more on this premium-priced product."
Tim Van der Schraelen, Beneo-Orafti's marketing and communication manager, concluded, "The results show that there is still a strong and very profitable market for those looking to create relevant functional foods."
The fact that health-conscious consumers are drawn to healthier-sounding foods is all the more reason why it's crucial for FDA to ensure that claims are made responsibly.
Claims Crackdown?
In a 2006 critique of an FDA report to Congress on NLEA enforcement, CSPI wrote, "If the Agency had the will to act, it could find the necessary resources to enforce the law."
The new administration does seem ready to wield a stronger arm. Many saw the increased number of warning letters and actions taken by FDA and the Federal Trade Commission last year against misleading claims as a good sign. As Campbell remarks, "[Last year's enforcement actions] are more than has happened in the last eight years."
Commissioner Hamburg says that she is embracing a stronger approach. In a letter to industry on March 3, she stated, "I have made improving the scientific accuracy and usefulness of food labeling one of my priorities."
In her letter, Hamburg also announced that in February, FDA had sent warning letters to 17 food manufacturers over label-claims violations. The letters were sent to brands including Gerber, Nestlé, Pom, and Sunsweet, and addressed infractions such as unauthorized health claims and nutrient-content claims.
Hamburg also said FDA is serious about establishing front-of-package labeling rules, and will soon release Draft Guidance on both front-of-package and nutrient labeling.
It remains to be seen whether FDA resources will allow the agency to fulfill its ambitions. Even if the agency's recent request of a 23% budget increase were granted, it's probable that food labeling would fall beneath other FDA priorities such as food safety and medical devices.
Meanwhile, Campbell thinks that the warning letters FDA has issued so far have been effective. "Very often, that's enough to make a company cease misleading labeling," she says. "And writing a warning letter to a company is less expensive than the next step, which would require getting a court involved. That's never cheap."
WHICH FRONT-OF-PACKAGE LABEL DO YOU LIKE BETTER?
AS PART OF ITS EFFORT to set standardized guidelines for front-of-package (FOP) labeling, the Food and Drug Administration (FDA) has launched a consumer research study to determine how consumers respond to various FOP nutrition label designs. (Below are a few labels the agency is currently testing with consumers.)
On March 3, FDA commissioner Margaret Hamburg announced that the agency will soon issue new Draft Guidance for both FOP and nutrition labeling.

View additional versions of the various front-of-package label designs that FDA is currently testing, online at: NutritionalOutlook.com/1003/labeling
Campbell says that she's waiting to see whether this administration's FDA will take us into an era of greater policing. "The pendulum is starting to swing back-but it's just starting to swing, and we don't know how far that swing is going to go. I personally hope we're seeing the pendulum swing back in the direction of increased enforcement."
Silverglade also believes that it's time for change. "I think we're at the bottom of the cycle, and things have gotten pretty darned bad out there in terms of deceptive claims."
Says Campbell, "I think there are some people pushing the envelope, and they're pushing it too far. If some of them got into trouble, then everybody else would toe the line better."
HHS announces restructuring plans to consolidate divisions and downsize workforce
Published: March 27th 2025 | Updated: March 27th 2025According to the announcement, the restructuring will save taxpayers $1.8 billion per year by reducing the workforce by 10,000 full-time employees and consolidating the department’s 28 divisions into 15 new divisions.
DOJ asks Utah court to dismiss FTC lawsuit against Xlear Inc.
March 11th 2025On March 10, the DOJ and the defendant filed a stipulation to dismiss with prejudice the lawsuit in which each party agrees “to be responsible for its own costs and fees and agrees that no party shall be responsible to any other party for any fines, costs, fees, or penalties arising from this case.”





